What is Float Glass?
The float system emerged by fifties, at Great Britain. The innovator process, created by sir Alastair Pilkington, was based on making the glass (or dough), not yet melted, floating on molten tin. Then, the glass gets the desired thickness, it is cooled and cut. Also known as flat glass, the floats are excellent uniformity and have almost no distortion.
The float glass process was developed by Pilkington by 1952 and it is a worldwide standard for manufacturing high quality glass.
The product, that originally produced only 6mm thickness glass, currently produces glasses with thickness that very between 0,4 e 25mm. The raw material are mixed with accuracy and merged in the oven. The glass merged to approximately 1000° C, is continuously shed in a tank full of liquid tin, chemically controlled. It floats on the tin, spreading it evenly.
The thickness is controlled by the speed of the glass sheet that solidifies as it keeps advancing. After annealing (controlled cooling), the process ends with the glass having polished and parallel surfaces.
Generally used in building, vehicles, home appliance and goods for decoration, the float glass are currently considered dominants in the glass industry all over the world.
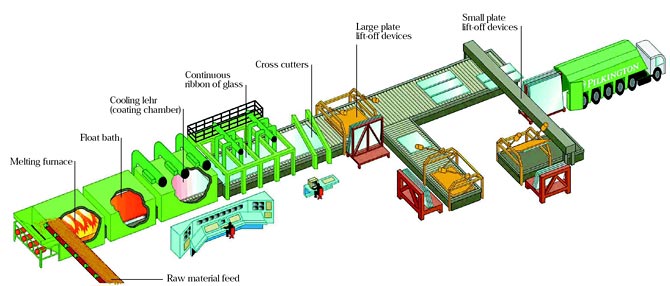
Description
The glass is an inorganic substance, homogeneous and amorphous, obtained by cooling molten dough. Its main qualities are transparency and hardness.
The glass is distinguished from other materials by various characteristics: it is not porous or absorbent, it is great insulator, has low expansion and thermal conductivity, it supports pressures from 5.800 to 10.800 kg per cm2.

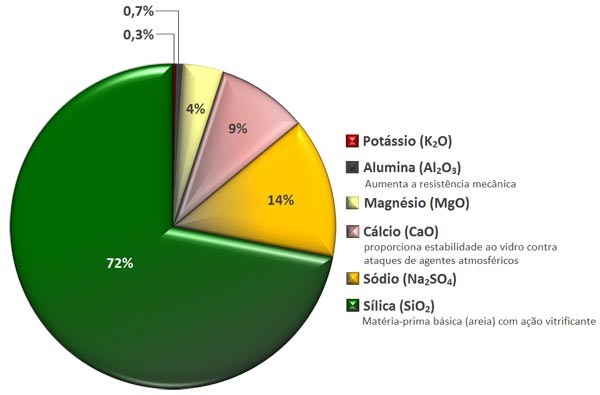
Chemical composition of the glass
The scrap glass, clean and selected, is used to assist in fusion. The colored glass are produced by adding some dye to the composition as selenium (Se), iron oxide (Fe2O2) and cobalt (Co3O4) to achieve different colors.
Main characteristics and qualities
- Recyclability
- Transparence (permeable to light)
- Hardness
- No absorbance
- Great electric insulator
- Low thermal conductivity
- Sources largely found in the nature
- Durability